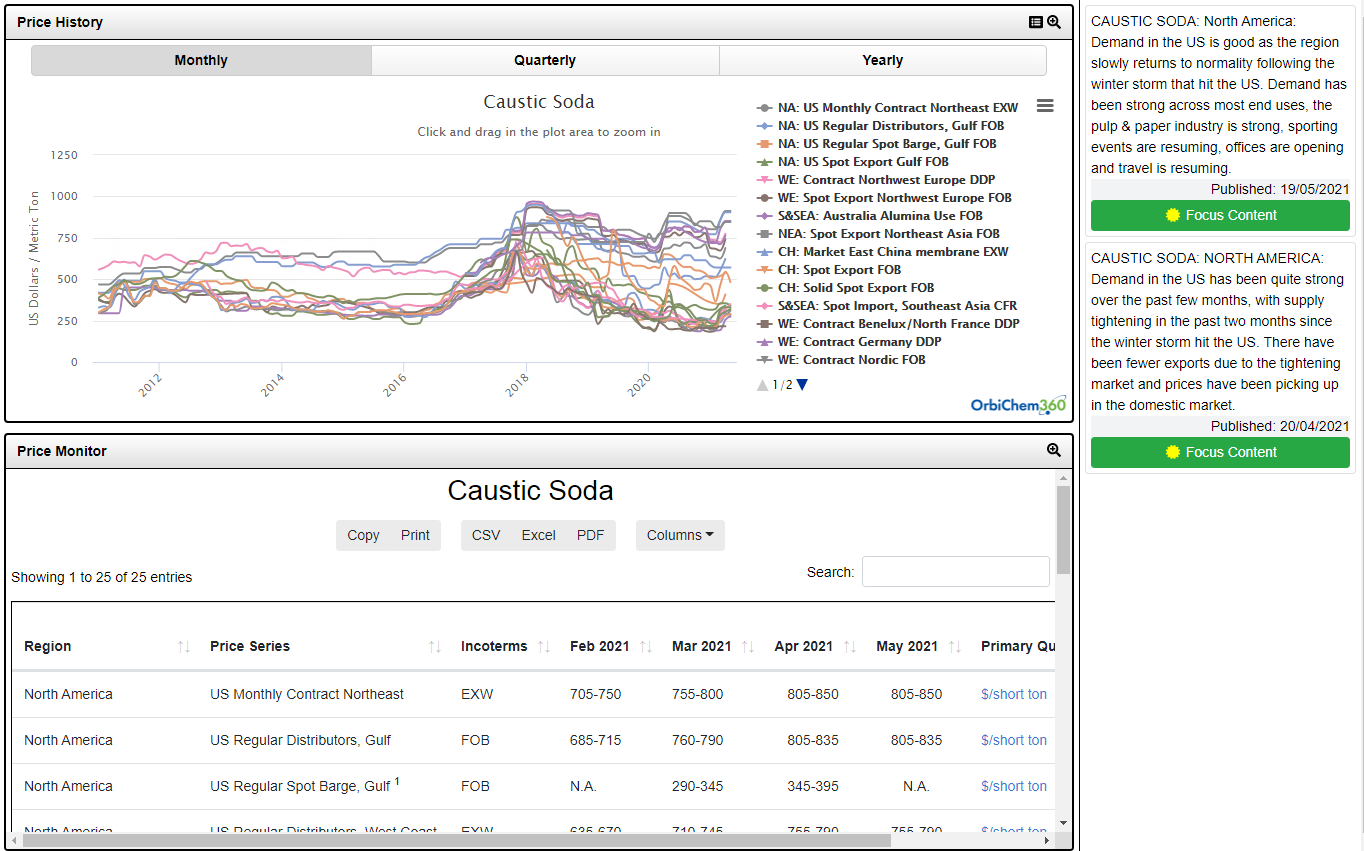
Caustic soda, or sodium hydroxide, is co-produced with chlorine by the electrolysis of sodium chloride, in the ratio of 1.1 tons of caustic soda for every 1.0 ton of chlorine. It has a wide range of consuming industries, including pulp & paper, textiles, soaps & detergents and bleach, where it is often used as a source of alkalinity. Another important consumer is the alumina industry that often attracts most attention due to its importance to global trade. Caustic soda can be sold in anhydrous form including pellets and flakes but the majority is sold as a 50% aqueous solution.
-
 Market Analysis
Market Analysis
-
 Prices
Prices
-
 Trade Data
Trade Data
-
 Market Summary
Market Summary
-
 Price Forecasts
Price Forecasts
-
 Supply/Demand
Supply/Demand
-
 Single Client Projects
Single Client Projects
The majority of chlorine is co-produced with caustic soda by the electrolysis of sodium chloride. Some processes allow the production of chlorine without the caustic soda and in some cases potassium hydroxide is the co-product. Chlorine is used in the production of many organic and inorganic derivatives although is often used only as a reactant and is not normally present in the final products. Large volumes of chlorine are consumed in the production of EDC, the pre-cursor for VCM used to make PVC.
-
 Market Analysis
Market Analysis
-
 Prices
Prices
-
 Trade Data
Trade Data
-
 Market Summary
Market Summary
-
 Price Forecasts
Price Forecasts
-
 Supply/Demand
Supply/Demand
-
 Single Client Projects
Single Client Projects
Hydrochloric acid (HCl) is available in both anhydrous (hydrogen chloride) and aqueous forms (also called muriatic acid). It is a by-product of many chemical processes involving chlorine. Hydrogen chloride can also be made deliberately by the reaction of chloride salts with sulphuric acid (Mannheim process), or through the reaction of hydrogen with chlorine at high temperature (chlorine burners). The latter is often called synthesis or burner HCl and being of high purity, can be used in food and electronics applications. The main markets for by-product hydrochloric acid include the oil and steel industries, while anhydrous HCl is used in chemical synthesis, for example to make dichloroethane. Since the generation of by-product HCl may well exceed future demand, there is growing interest in processes that can convert HCl back to chlorine.
-
 Market Analysis
Market Analysis
-
 Prices
Prices
-
 Trade Data
Trade Data
-
 Market Summary
Market Summary
-
 Price Forecasts
Price Forecasts
-
 Supply/Demand
Supply/Demand
-
 Single Client Projects
Single Client Projects
MDI or methylene diphenyl diisocyanate is an important raw material for polyurethanes. MDI undergoes a series of complex manufacturing stages. The process starts with benzene and nitric acid, and via aniline and formaldehyde reactions is also subsequently phosgenated to the final mix of isomers, which are distilled to the desired series of end-products. MDI is used in polyurethane chemistry by reaction with polyols, to produce a wide range of flexible and rigid foams, and elastomers, adhesives and coatings.
-
 Market Analysis
Market Analysis
-
 Prices
Prices
-
 Trade Data
Trade Data
-
 Market Summary
Market Summary
-
 Price Forecasts
Price Forecasts
-
 Supply/Demand
Supply/Demand
-
 Single Client Projects
Single Client Projects
TDI or toluene diisocyanate is a key raw material for polyurethane applications. The starting point for TDI is toluene and nitric acid, which thereafter undergo phosgenation reactions as well as distillation to arrive at the final range of TDI isomers. TDI is used in polyurethane chemistry by reaction with polyols, to produce a wide range of applications. TDI is produced in different grades, either for slab polyurethane foam which makes up the major end use sector, or for coatings adhesives sealants and elastomers applications. Flexible foam is largely used in mattress and furniture production.
-
 Market Analysis
Market Analysis
-
 Prices
Prices
-
 Trade Data
Trade Data
-
 Market Summary
Market Summary
-
 Price Forecasts
Price Forecasts
-
 Supply/Demand
Supply/Demand
-
 Single Client Projects
Single Client Projects
Propylene oxide (PO) is a chemical intermediate produced from propylene. Traditionally two methods involving hydrochlorination and the other involving oxidation were employed. PO can be produced with styrene monomer as a coproduct (POSM process). More recently, a new process known as HPPO in which propylene is oxidized with hydrogen peroxide was introduced commercially. The main end use is in the manufacture of polyether polyols which are used in conjunction with isocyanates to produce polyurethanes. The second major use is in the production of propylene glycols. Other uses include the production of glycol ethers and ,in some cases, butanediol.
-
 Market Analysis
Market Analysis
-
 Prices
Prices
-
 Trade Data
Trade Data
-
 Market Summary
Market Summary
-
 Price Forecasts
Price Forecasts
-
 Supply/Demand
Supply/Demand
-
 Single Client Projects
Single Client Projects
Epichlorohydrin (ECH) is a chlor-epoxide that is essentially based on the reaction of chlorine with propylene through a number of reaction steps: it may be regarded as being similar to propylene oxide with the addition of a reactive chlorine site. The two routes to ECH are via allyl chloride and allyl alcohol: allyl chloride is by far the main route. ECH is used mainly (70%) in the production of epoxy resins and also in the manufacture of synthetic glycerine and cationic polymers and starches.
-
 Market Analysis
Market Analysis
-
 Prices
Prices
-
 Trade Data
Trade Data
-
 Market Summary
Market Summary
-
 Price Forecasts
Price Forecasts
-
 Supply/Demand
Supply/Demand
-
 Single Client Projects
Single Client Projects
Ethylene is one of the major chemical building blocks and the largest of the olefins (by sales volume). Its main use is as the monomer for various forms of polyethylene. Ethylene is produced by steam cracking, predominantly of naphtha in Europe and Asia, and of ethane in North America and the Middle East. Co-products are propylene and a C4 stream containing butadiene. Ethylene is also used to produce vinyl acetate and as a co-monomer in other types of resin. Although propylene is growing in importance, the demands of the ethylene market still mainly drive the operation of steam crackers.
-
 Market Analysis
Market Analysis
-
 Prices
Prices
-
 Trade Data
Trade Data
-
 Market Summary
Market Summary
-
 Price Forecasts
Price Forecasts
-
 Supply/Demand
Supply/Demand
-
 Single Client Projects
Single Client Projects
EDC is the primary intermediate feedstock in the production of polyvinyl chloride (PVC). EDC is in turn made from ethylene and chlorine. The use of EDC in the manufacture of PVC covers most of world production: the remainder uses a route via acetylene and is only used in China. The majority of EDC is used for PVC manufacture.
-
 Market Analysis
Market Analysis
-
 Prices
Prices
-
 Trade Data
Trade Data
-
 Market Summary
Market Summary
-
 Price Forecasts
Price Forecasts
-
 Supply/Demand
Supply/Demand
-
 Single Client Projects
Single Client Projects
VCM is an intermediate feedstock in the production of polyvinyl chloride (PVC). It is usually made by cracking ethylene dichloride (EDC) itself made from ethylene and chlorine. However, in China most VCM is from acetylene (via calcium carbide), particularly in the remote northern and western provinces.
-
 Market Analysis
Market Analysis
-
 Prices
Prices
-
 Trade Data
Trade Data
-
 Market Summary
Market Summary
-
 Price Forecasts
Price Forecasts
-
 Supply/Demand
Supply/Demand
-
 Single Client Projects
Single Client Projects
A thermoplastic ultimately derived from ethylene and chlorine via the intermediates ethylene dichloride (EDC) and vinyl chloride monomer (VCM). Uses are split in two types: “Rigids” which contain no plasticisers and “Flexibles” which do. The largest single use is for a wide variety of PVC pipes for sewage, drainage, potable water etc.
-
 Market Analysis
Market Analysis
-
 Prices
Prices
-
 Trade Data
Trade Data
-
 Market Summary
Market Summary
-
 Price Forecasts
Price Forecasts
-
 Supply/Demand
Supply/Demand
-
 Single Client Projects
Single Client Projects
Carbon tetrachloride (CTC), more correctly called tetrachloromethane, is an organic compound with the chemical formula CCl4. It was formerly widely used in fire extinguishers and as a cleaning agent, but such uses, which allow evaporation into the atmosphere, have been banned under the Montreal Protocol. Also the use of carbon tetrachloride to produce the chlorofluorocarbon refrigerants R-11 (trichlorofluoromethane) and R-12 (dichlorodifluoromethane) has been phased out, since these refrigerants play a role in ozone depletion. As a result of this phase out, many CTC plants had to shut down, but some consumption and production continued, because use of CTC to make chemical derivatives (so called non-controlled uses) is allowed. Some hydro-fluorocarbons (HFCs) use CTC in their manufacture, but today a number of HFCs are considered to have an unacceptably high Global Warming Potential (GWP). The Kigali Amendment in November 2017 modified the Montreal Protocol by adding HFCs to the list of regulated substances. Starting in 2019, some HFCs, such as HFC245fa and HFC410A, must be phased out over time. The most likely safe and economic substitutes will by hydrofluoro-olefins (HFOs), which are well inside the GWP threshold. Some of them require CTC as feedstock, so a resurgence in its use is likely.
-
 Market Analysis
Market Analysis
-
 Prices
Prices
-
 Trade Data
Trade Data
-
 Market Summary
Market Summary
-
 Price Forecasts
Price Forecasts
-
 Supply/Demand
Supply/Demand
-
 Single Client Projects
Single Client Projects
Chlorobenzenes represent a family of products based on the reaction of benzene with chlorine. They are mainly used as chemical intermediates in the production of advanced polymers, in the manufacture of agrochemicals, and via nitrochlorobenzenes in the production of dyestuffs and pigments.
-
 Market Analysis
Market Analysis
-
 Prices
Prices
-
 Trade Data
Trade Data
-
 Market Summary
Market Summary
-
 Price Forecasts
Price Forecasts
-
 Supply/Demand
Supply/Demand
-
 Single Client Projects
Single Client Projects
The chloroethanes group of products includes trichloroethylene (solvent and intermediate), perchloroethylene (solvent and intermediate), 1,1,1-trichloroethane (intermediate), ethyl chloride (intermediate), and vinylidene chloride (VDC) (intermediate). They are generally based on the reaction of chlorine or hydrochloric acid with hydrocarbons or chlorocarbons in the range C1-C3 and have complex production economics based on the feedstocks used. The intermediate uses for trichloroethylene, perchloroethylene and 1,1,1-trichloroethane are in fluorocarbons. Ethyl chloride is used mainly in tetraethyl lead and ethyl cellulose. VDC’s main use is to make the polymer polyvinylidene chloride but it is also used in fluorocarbons and to make 1,1,1-trichloroethane.
-
 Market Analysis
Market Analysis
-
 Prices
Prices
-
 Trade Data
Trade Data
-
 Market Summary
Market Summary
-
 Price Forecasts
Price Forecasts
-
 Supply/Demand
Supply/Demand
-
 Single Client Projects
Single Client Projects
Chloromethanes is a collective name for the four products that arise from the reaction of chlorine with methane or methanol. These are methyl chloride; methylene chloride; chloroform; and carbon tetrachloride. Methyl chloride is a gas that is largely produced on site as a feedstock by its main consumers in the silicones industry and for chlorination to the other chloromethanes. The main commercially traded products are methylene chloride, which is primarily used as a solvent in industries such as pharmaceuticals, surface cleaning, and chemical processing; and chloroform, which is mainly used as a chemical intermediate to produce fluorochemicals. Carbon tetrachloride is not allowed for use as a solvent but has applications as a chemical intermediate for fluorocarbons and agrochemicals.
-
 Market Analysis
Market Analysis
-
 Prices
Prices
-
 Trade Data
Trade Data
-
 Market Summary
Market Summary
-
 Price Forecasts
Price Forecasts
-
 Supply/Demand
Supply/Demand
-
 Single Client Projects
Single Client Projects
Monochloroacetic acid is a chemical intermediate largely produced from acetic acid and chlorine although there are other routes. Its main uses are in the manufacture of carboxy methylcellulose, of a number of agrochemicals including glyphosate, of mild surfactants, of polymer stabilisers and various other applications.
-
 Market Analysis
Market Analysis
-
 Prices
Prices
-
 Trade Data
Trade Data
-
 Market Summary
Market Summary
-
 Price Forecasts
Price Forecasts
-
 Supply/Demand
Supply/Demand
-
 Single Client Projects
Single Client Projects



 ChemFocus
ChemFocus ChemFacts
ChemFacts ChemForesight
ChemForesight ChemExpert (includes ChemFocus modules)
ChemExpert (includes ChemFocus modules) Consulting
Consulting